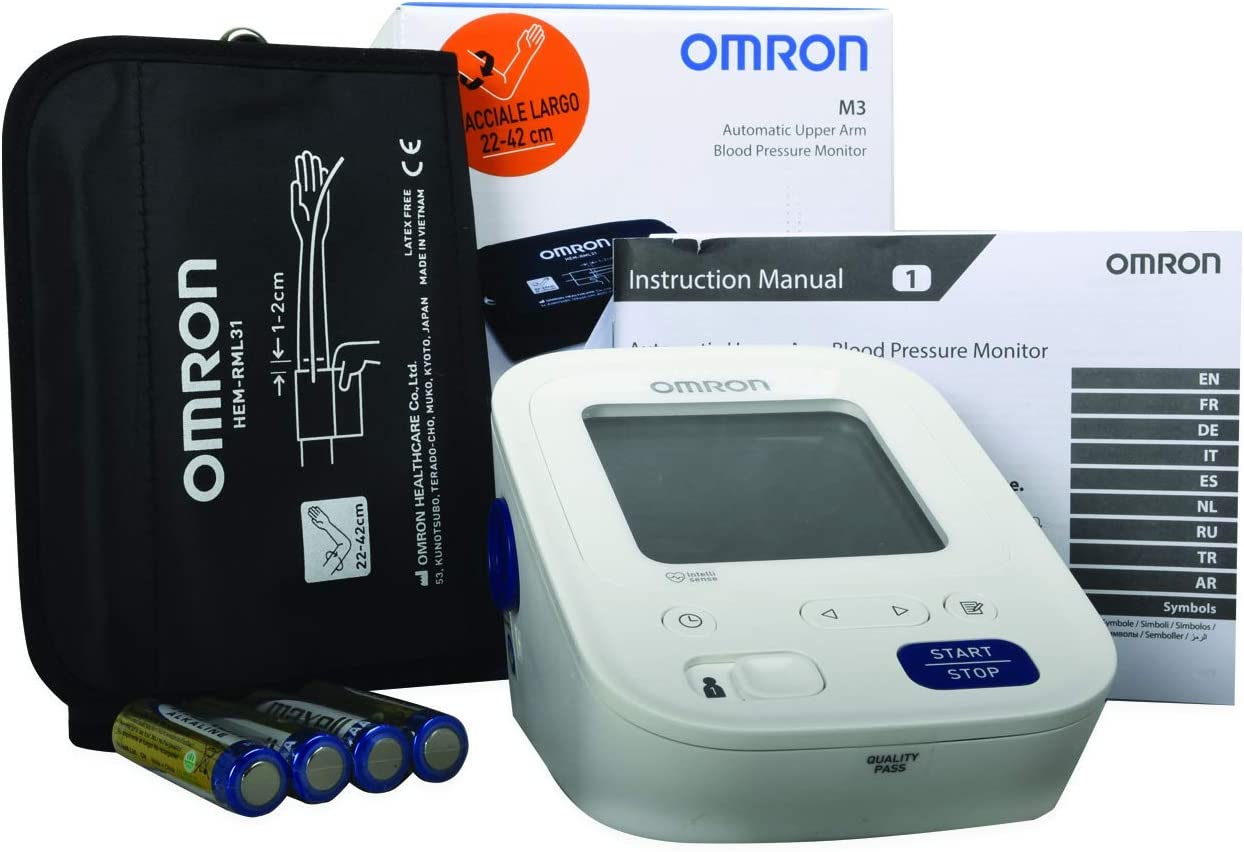
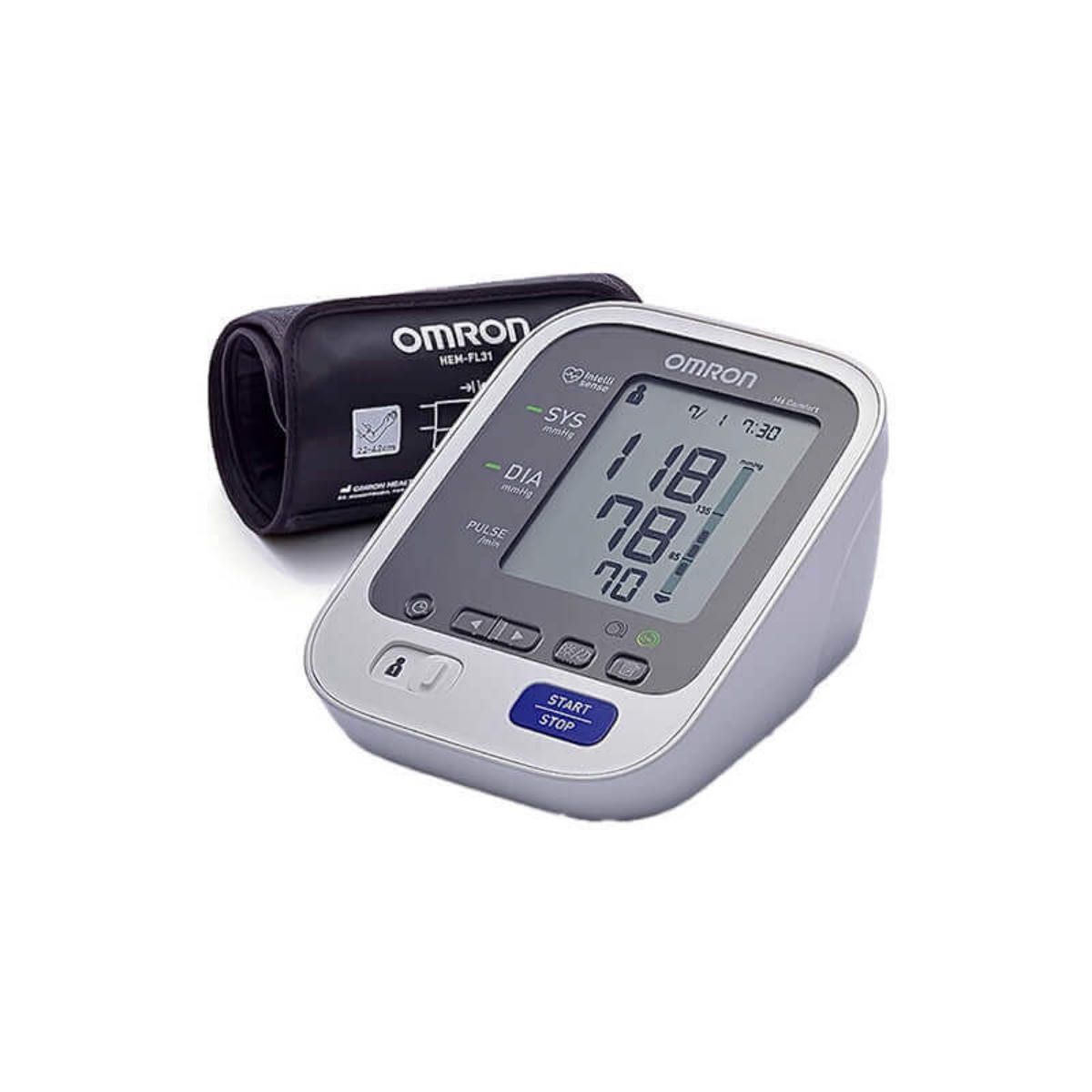
اشترِ اومرون كمفورت جهاز قياس ضغط الدم من أعلى الذراع HEM-7134-E M3 عبر الإنترنت في الإمارات العربية المتحدة | شرف دي جي

جهاز قياس الضغط Omron M3 ⠀⠀⠀⠀⠀⠀⠀⠀⠀ •⠀⠀⠀⠀⠀⠀⠀⠀⠀ قياس ضغط الدم بالمنزل بطريقة صحيحة وبكل دقة⠀⠀⠀⠀⠀⠀⠀⠀⠀ •⠀⠀⠀⠀⠀⠀⠀⠀⠀ من المستحيل وضع السوار بطريقة خاطئة، وذلك... | By Viomedica | Facebook
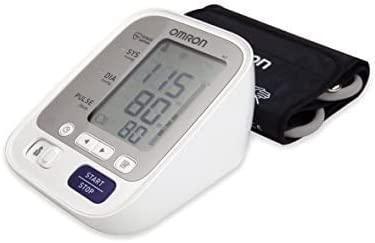
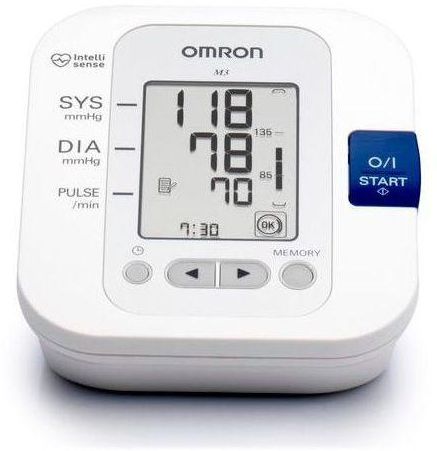
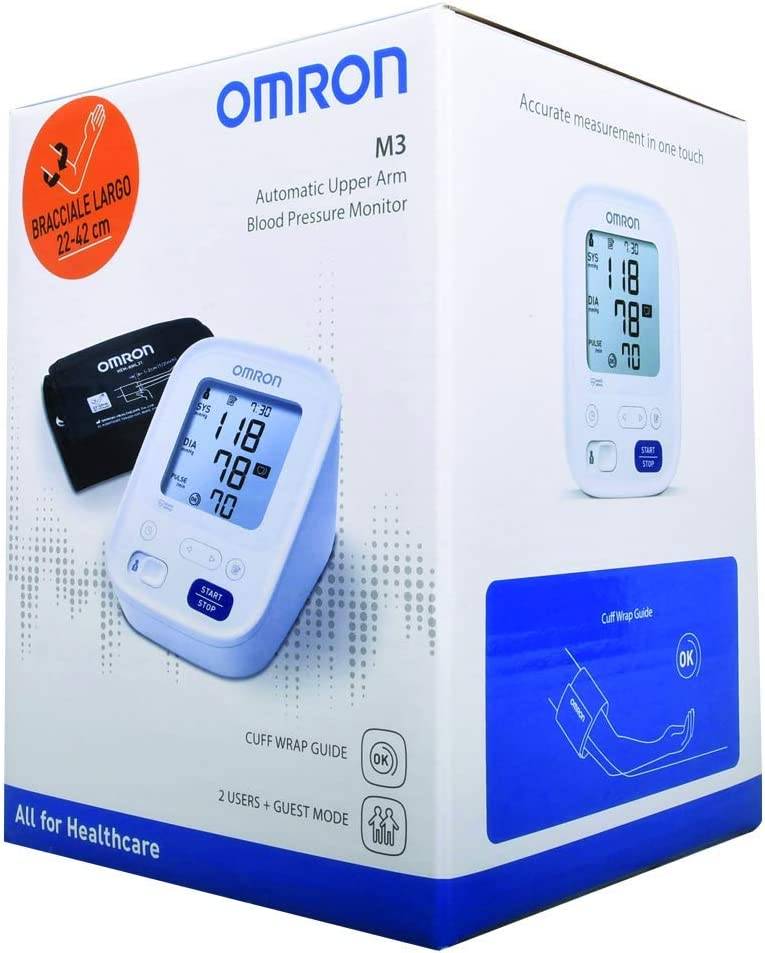
اطلب جهاز قياس ضغط الدم اومرون ام 3 رقمي محمول Omron M3 Automatic Blood Pressure Monitor الأصلي | Jomla.ae

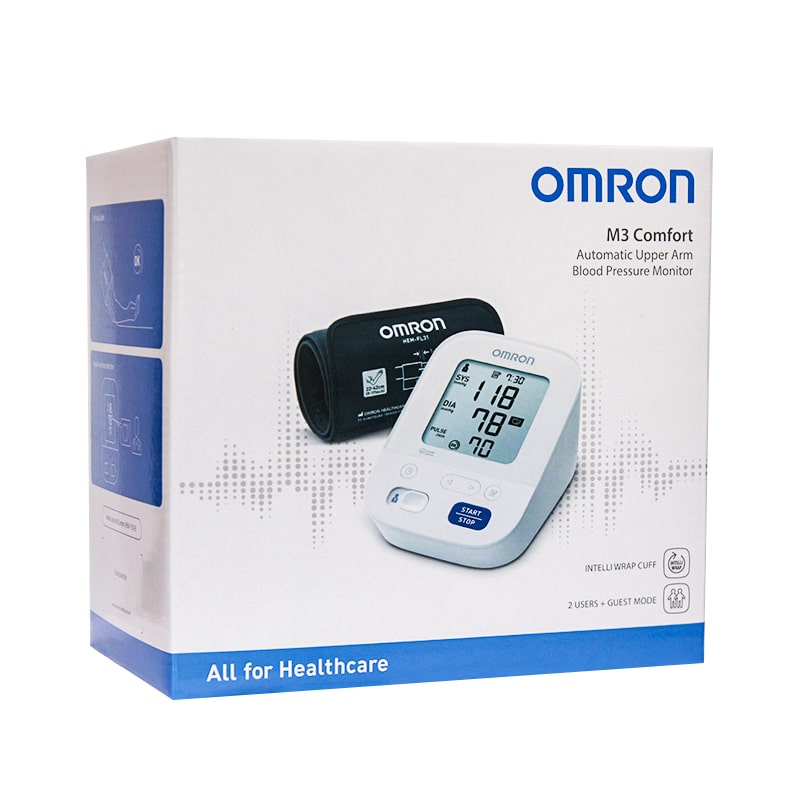
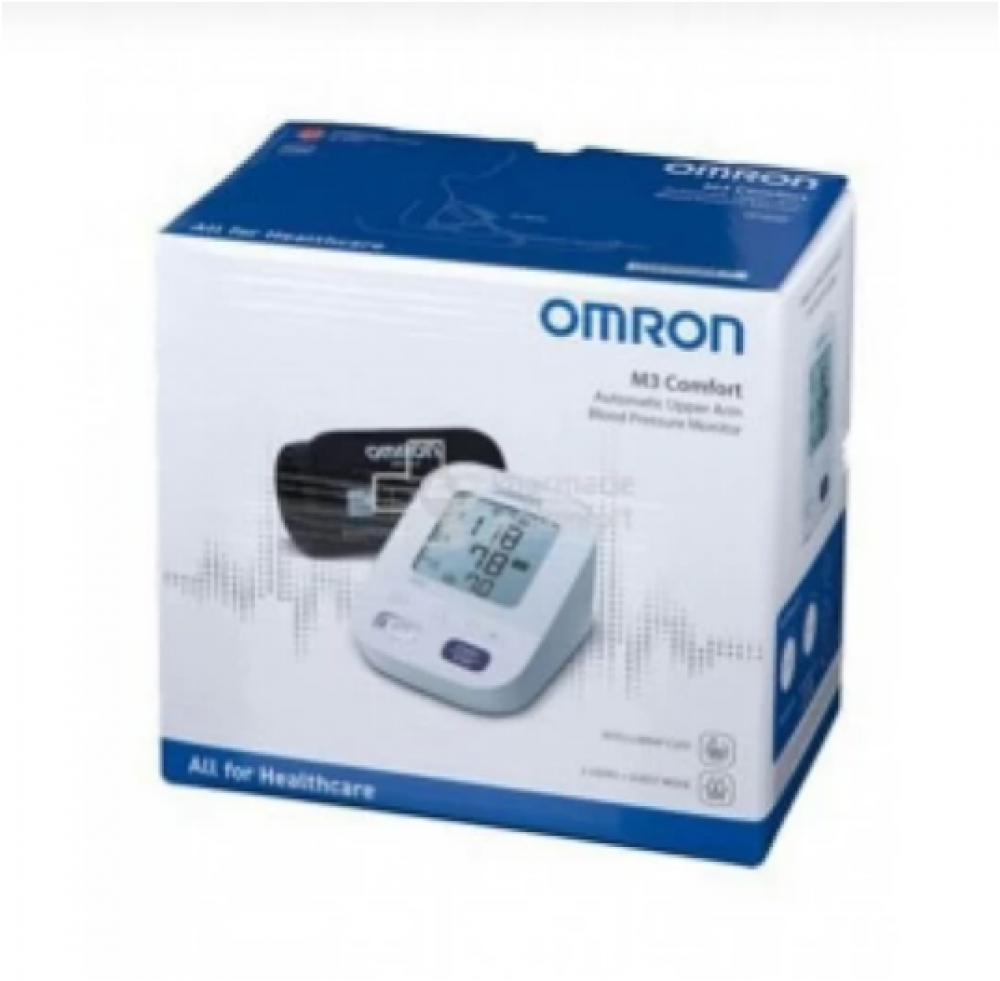
قم بشراء أومرون جهاز قياس ضغط الدم M3 + أمرون مقياس الحرارة + شاحن Online at Best Price من الموقع - من لولو هايبر ماركت BP Monitor

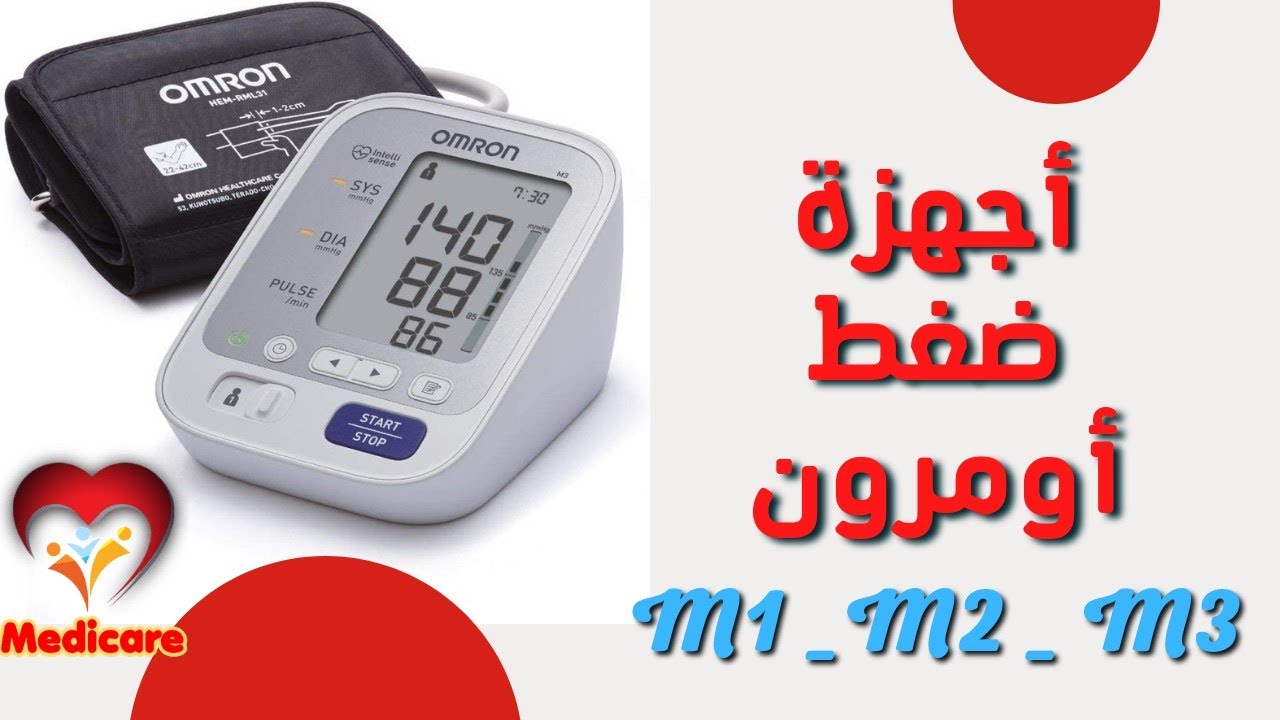
اشتري اونلاين بأفضل الاسعار بالسعودية - سوق الان امازون السعودية: جهاز قياس ضغط دم اومرون M3 لاعلى اليد : الصحة

LEE. omron-m3-blood-pressure-monitor souq.com. awok.com. noon.com. sharafdg.com. ubuy.ae. namshi.com. mumzworld.com. letstango.com